Pulsed compression technology
Processes for production of bulk chemicals at high temperatures and pressures account for major part of energy use and emissions in chemical and petrochemical industries. The main reason is that combustion of expensive fossil fuels – natural gas and oil – is used to generate high temperatures. Cooling of high temperature products is a serious technological problem since recovering high temperature energy requires expensive equipment and is highly inefficient – about 30% of the energy is lost. Temperature in industrial processes usually does not exceed 15000C (at low pressure) and pressure – 300 bar (at relatively low temperature) due to materials constraint. As a consequence, the production of liquid fuels and chemicals from natural gas (the world’s most abundant petrochemical resource), oil shale, coal, and biomass is not economic.

The world’s huge reserves of natural gas most of which are in remote locations, a long way from potential markets, are not used because transportation of the gas is not economic. An alternative technology to make the fuels and feedstocks from the gas in situ and then transport these high-value finished products to their ultimate destinations does not exist.
Industrial processes that use methane as a chemical feedstock for hydrogen and synthesis gas production are energy and capital intensive and generate enormous amount of CO2. Known processes for methane conversion to olefins and hydrogen – cracking in electric arc, oxidative pyrolysis, cracking in shock waves, thermal and oxidative coupling – are not economic. The most important bulk chemicals – ethylene and propylene – are produced from limited feedstocks – naphtha, ethane, gas oil, propane.
In recent years Encontech in close cooperation with the University of Twente have shown the feasibility of pulsed compression technology. It has been shown that the production of synthesis gas from methane, by partial oxidation, is possible in a free piston pulsed compression reactor. This reactor consists of a cylinder with a reciprocating piston between two reaction chambers (see Figure 1).
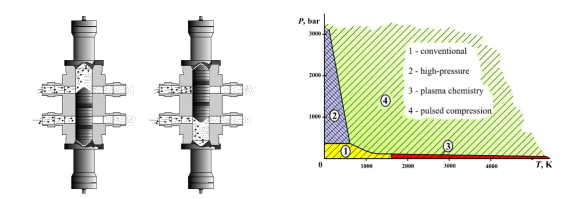
With the compression technology chemical transformations can be carried at temperatures and pressures which have never been thought not only in industry but even in experimental practice as is shown in Figure 2. Gas temperature and pressure during compression by a solid piston can be as high as 10000 K and 10000 bar.
It was also shown that the technology is applicable for carrying our many industrially important processes such as steam methane reforming and carbon dioxide methane reforming. Also methane cracking was studied. If a mixture of methane with argon is subjected to rapid compression-expansion, then it is converted to valuable chemicals – ethylene, acetylene, hydrogen. Methane conversion was about 80%, half of the cracked methane was converted to ethylene and acetylene. No other methods provide better results.
Feasibility has been shown and results have been published in literature. Reactants are heated by compression at a very high heating rate (107 K/s) to very high temperatures (3000 K) and pressures (700 bar), followed by a very fast cooling rate (107 K/s).
An extremely nice feature of this reactor is that the process of heating and cooling is very close to reversible. In conventional reactors fossil fuels are combusted for heating, whereas in the pulsed reactor reversible rapid compression is used for heating and accelerating chemical reactions.
The description of the free piston pulsed compression reactor is presented in the Technology Report.
Free piston pulsed compression reactors can potentially cover only very rapid gas-phase chemical reactions. They cannot be used for relatively slow or multiphase reactions, especially containing solid particles such as heterogeneous catalytic reactions due to inevitable problems with wear and sealing of the piston in the cylinder under the severe conditions of a combination of high temperature, pressure and sliding speed. Inherent problems of these reactors are short circuit losses and mixing of products and feed. Residence time in the free piston reactors depends on feed initial pressure and mass of the piston.
To overcome the restrictions and shortcomings of the free piston pulsed compression reactors radically new chemical reactors – Liquid Compression Chemical Reactors – have been proposed by Encontech. The main difference of the proposed reactors compared to the free piston reactors is that compression of reactants is achieved by a liquid (salt melts solutions, liquid metals).